Strong investment in process and product development and research is Nornickel’s key to future success
We set far-reaching goals and adapt to an ever-changing operating environment.
Maintaining the efficiency of production processes, improving their economic efficiency and ensuring environmentally sound production require continuous research and development. Our research department’s chemistry and metallurgy professionals work to discover better solutions and to utilize nickel even more efficiently.
We keep continuously developing and modernizing our operations. Our research teams also collaborate with the Group’s other research and development teams and the technical customer service team. Our development work is guided by the needs of our customers. Their feedback has a major effect on research and development activities. Practical feedback makes it possible to develop and refine our products and to make them even more suitable for the customers’ needs.
LION
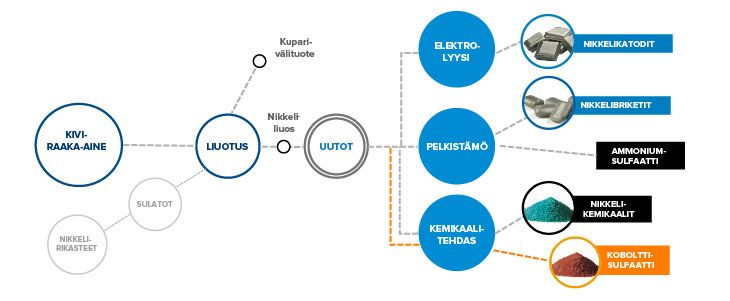
Pyrometallurgical and precipitation processes produce nickel, cobalt and copper-containing raw materials that are dissolved from solids into an aqueous solution by leaching. The solution form of nickel is required for further processing. The metal-containing fines are ground in ball mills. The water is separated and the ground fines are dissolved using sulfuric acid and oxygen.
After atmospheric reactor leaching, the insoluble solids are passed to high-temperature pressure leaching in autoclaves. This process dissolves the nickel contained in the fines and separates impurities such as iron and copper.
The raw nickel solution, which also contains cobalt, is pumped further to the extraction section for fine purification. The leaching also produces an intermediate product, a copper precipitate with a high copper content containing precious metals, which is sold for further copper refining.
EXTRACTS
The raw nickel solution from the leaching plant is cleaned of impurities, such as cobalt, in the leaching and sulfide precipitation sections of the reduction plant. The raw nickel solution from the leaching plant for cobalt extraction is cooled and finely filtered to prevent foreign organic and solid substances from entering the extraction process.
Cobalt extraction is carried out in sequential cobalt extraction cells, where the raw nickel solution flows countercurrently to the extraction solution. In the extraction process, in addition to cobalt, other impurities, including copper, zinc, iron and manganese, are transferred from the nickel solution to the organic extraction solution.
The nickel sulfate solution from the leaching process first passes partly through the calcium extraction, where in addition to calcium, iron, zinc, copper and manganese are extracted from it, after which it is pumped to the cobalt extraction.
After solution purification, part of the purified nickel solution is sent to electrolysis and part to the reduction plant for further processing. In solution purification, cobalt is separated as a raw cobalt solution, which is further purified before the solution is transferred to a chemical plant for the production of cobalt sulfate.
REDUCTION
Nickel briquettes are produced in the reduction plant. Hydrogen reduction of the purified nickel solution is carried out in batches under suitable conditions using hydrogen gas in autoclaves. The nickel powder produced in the autoclaves is separated from the solution by settling and filtering. After drying, the nickel powder is transferred to a powder silo for briquetting or to a powder packaging silo for packaging for customers. The powder is briquetting mechanically and the resulting briquettes are transferred to nitrogen sintering, the purpose of which is, among other things, to strengthen the briquettes by heating.
The finished nitrogen-sintered briquettes are packaged for delivery to customers. The ammonium sulfate raw solution generated during nickel reduction is led to a precipitation unit for purification, where the small amount of nickel remaining in the solution is separated and returned to the process. After precipitation, the ammonium sulfate solution, purified from nickel, is crystallized and dried into a product for sale as a fertilizer.
ELECTROLYSIS
The purpose of electrolysis is to produce cathode nickel electrolytically. The production is carried out using the electrowinning method, in which direct current is fed through an insoluble lead anode into the electrolyte and further to the Ni cathode, to which nickel is transferred from the nickel solution by means of an electric current.
The Ni cathode is placed inside a diaphragm bag, where the nickel solution prepared in the dissolution plant and purified in the extraction plants, i.e. catholyte solution, is fed. In the electrolysis process, Ni ions are reduced from the solution to the surface of the cathode by means of an electric current. The circulation or growth time of the cathodes in the electrolysis tanks is approximately seven days. The amount of reduced nickel depends on the current, current efficiency and the number of tanks in the circuit. The cutting plant carries out the cutting, packaging and loading of the cathode nickel.
CHEMICAL FACTORY
The chemical plant produces inorganic salts: sulfates, hydroxycarbonates and hydroxides from the highly pure nickel sulfate solution obtained from the extraction. The department has its own production lines for nickel sulfate, nickel hydroxycarbonate and nickel hydroxide. In addition to nickel products, the chemical plant crystallizes cobalt sulfate crystals from the purified cobalt sulfate solution.
Ni-Sulphate lines produce STD (standard) and EN (electroless nickel) quality nickel sulphate crystals. Hydroxycarbonate lines produce so-called dry, paste and granule products. The hydroxide line produces STD and HD quality hydroxide.
In the sulfate line, the solution is crystallized by evaporation in a continuous crystallization process. The crystals are dried, screened, stored in silos and packaged.
In a continuous hydroxycarbonate line, nickel is precipitated from the solution with soda. The resulting precipitate is filtered, washed, dried, stored in silos and packaged. In a hydroxide line, hydroxide is precipitated with lye. The resulting precipitate is filtered, washed and dried. Finally, the product is stored in silos and packaged. Pure cobalt sulfate crystals are produced in a crystallization process, similar to nickel sulfate, dried, screened and packaged for further delivery to customers.



